About lake Taihu: A large, shallow and turbid lake
geographical location of the lake in the yangtze delta plain
 Taihu, 2004:
Taihu, 2004:
View onto the
open lake, not
far from the Meiliang Bay near city Wuxi.
 Catchment of Taihu, city Wuxi,
1994:
Catchment of Taihu, city Wuxi,
1994:
Busy traffic on the Grand Canal - watching the plenty of barges being
aligned to chains.
Lake Tai (Taihu) is located on the Yangtze Delta plain in China (31°15’28.6''N, 120°12’18.39''E), at 7 m above sea level. The subtropical lake has a surface area of 2338 km2 and a huge catchment area of 36500 km2. Wuxi is the largest city on Taihu, in the north of the lake, at the Meiliang Bay. Taihu is the third largest freshwater lake in China. The shallow lake has a maximum depth of about 4.5 m and a mean depth of 2 m. The lake water of volume of 4.44 x 109 m3 has a relatively short retention time of less than one year only (264 days, Table 1 in Chen et al. 2003 R). The seasonal water level fluctuations are relatively low and the water is less turbid by inorganic silt particles when comparing Taihu with Poyang Lake S. The water level of Poyang Lake, which is direct connected with the Yangtze River, can vary from 8 to 19m as e.g. reported by Liu et al. (2016) R for the study period from Sept 2011 to December 2012.
The northern part of the lake and
the large Meiliang Bay:
farming in the north of the lake has changed a lot in the last two
decades.
This section is primarily intended to describe the photographs in the top gallery and text documenting the lake and the shore area in the years 1994, 1995, 2000, 2004 and 2011. The aspects discussed on this website for Taihu relate mainly to the phytoplankton species in the nutrient-rich northern part and describe the control of their growth by nutrients. The main focus is here on the planktonic cyanobacteria, which are common in this part of the lake. As mentioned elsewhere in this website, the coupling of nutrient enrichment and blooming of cyanobacteria in the water body is not a particular situation in a one lake only, but is a common phenomenon of eutrophied lakes across all the continents of the world. Examples of cyanobacterial blooms in this website are hence described not only for Taihu in Asia, but also for deep and shallow lakes in Europe (see certain years of phytoplankton development in deep Mondsee S and Ammersee S; and shallow Grosser Mueggelsee S).
Most of the 50 Taihu photos shown in the gallery are taken from the northern part of the lake, nearby or in the Meiliang Bay. Some lake photos are taken from a particular plankton situation in the year 2000, when cyanobacteria were drifted to shallow areas of the Meiliang by wind fetches (photos 30 to 34and 39 to 44). During other seasons or in years of lower nutrient concentrations, such a heavy scum of cyanobacteria might not be necessarily developed. Other gallery photos from Taihu (see photos 1 to 8, 11 to 17) illustrate a brownish turbid water body, which goes hand in hand with the high amount of inorganic suspended solids in this lake (see details in the paragraph below). To say it once again: Photos of the scum of cyanobacteria taken in early autumn in 2000, describe a very particular phytoplankton situation that is not common throughout all seasons a year and that might not necessarily be a typical situation for every autumn each year in Taihu.
 Town Wutang at Taihu, 1994:
Town Wutang at Taihu, 1994:
Work on rice fields. This photo is taken in the same area as
shown in the other three photos.
 Town Wutang at Taihu, 1995:
Town Wutang at Taihu, 1995:
Rice fields. Insert shows maturing ears of rice plants.
 Town Wutang at Taihu, 2000:
Town Wutang at Taihu, 2000:
Rice fields as in 1995, but surrounded by soybeans.
 Town Wutang at Taihu, 2011:
Town Wutang at Taihu, 2011:
Orchards and vegetable gardens replaced here the rice
fields. The photo shows the same landscape view as the photos with rice
fields taken in 1995 and 2000 (see the photo above and on left side).
There is growing awareness about cyanobacterial blooms in eutrophic lakes. In many eutrophied lakes, lake management successfully reduced the nutrient concentrations preventing the development of cyanobacteria all over the world (see example Old Danube on this website, S). Some photos in the Taihu gallery illustrate few aspects of bio-manipulation that aim at improving the lake water quality. These photos of lake management focus on harvesting plant material to transfer ‘natural’ macrophytes to other bay areas (photo 48) or on culturing flowers on floating platforms (photo 49, 50 and photo inserted in text below).
The lake is used by local fishery to get fish, freshwater shrimp and even mollusc that all is used for food. As fishing activities change from season to season in the year, only a one aspect could be illustrated during a one visit. Some visits were during seasons when freshwater shrimps were caught (photos 17&18 taken in 1994/1995). Other visits were during seasons of net catching the fish (e.g. photos 2 to 8 taken in 2004). A change with time over years might be seen from local fishery in the Meiliang Bay. While in the nineties, the Taihu shoreline was somehow ‘wild untouched’ and fish catch was simply dried outdoor on the shore (photo 20 and 21 taken in 1995 and 1994), a decade later the lakeshore area ends at a concrete wall with new areas of land reclamation (photo 22 taken in 2004).
Beside lake Taihu, the catchment of the lake is illustrated by further 20 photos in the gallery (photos 51-70). Most of these photos are taken from Wutang, a town that is close to the shoreline of Taihu. The Taihu-research station of the Chinese Academy of Sciences is nearby this town. Visits from time to time over a period of many years allowed seeing the change of the land use in the lowland of Taihu. While in the nineties just ‘pure’ rice fields were common, these fields became later surrounded by soybean belts and were finally replaced by orchards, individual vegetable gardens or even small vineyards. To illustrate these changes in the land-use, a series of photos from a same place, from a same landscape scenery taken over years were mainly selected (gallery photos 57-70). Inserted symbols mark here particular houses, buildings and the brickwork nearby the town Wutang to make it easier to identify the location on the photos. These symbols were only added to medium-sized photos. The location on the large photos is described by the photo title.
 Town Wutang at Taihu in
2011:
Town Wutang at Taihu in
2011:
Tea
plantation and tea pickers.
 Town Wutang at Taihu in 2011:
Town Wutang at Taihu in 2011:
Tea pickers after having finished the work of the day.
 Town Wutang at Taihu in
2011:
Town Wutang at Taihu in
2011:
The work in the tea factory.
 Town Wutang at Taihu in 2011:
Town Wutang at Taihu in 2011:
A farmer is selling tea on the local market.
In addition to rice field, some photos were selected showing orchards and plantations of peaches and oranges, as this agriculture use was moreover common for decades in this lowland area of Taihu. Additionally, a few photos were selected showing tea plantations, as this Taihu shore area is further famous for green tea (see the four photos above in the text).
high turbidity of the water of lake
taihu:
floating
particles of fine inorganic material and of small
biota.
 Turbidity of Taihu lake water,
2004:
Turbidity of Taihu lake water,
2004:
the
milky-brownish dark green water colour is mainly due to suspended
solids of small inorganic particles and phytoplankton.
Due to the shallowness and large surface, the
lake water is frequently mixing
from the surface to bottom in all seasons, induced by turbulences in
the water body and wind (polymictic
lake). High amounts of suspended
solids, of both organic (as e.g. phytoplankton cells) and
floating inorganic particles, are dispersed in the water column due to
mixing, which reduces water transparency (Fig.2
in Chen et al.
2003 R).
As described for other shallow lakes (see Old Danube and
Grosser Müsselsee), the nutrient-input and consequently, the yield of
biomass of micro-organisms as cyanobacteria and algae may tend to
increase in years of ‘eutrophication’ by urbanization and other
disturbances of natural lakes, but also can turn to declining trends in
years of ‘re-oligotrophication’ by lake restoration treatment. For
lakes that underwent or still undergo large ecosystem changes due to
nutrient-enrichment or nutrient reduction, it is hence necessary to
state clearly, from what period the study is. Further, for many reasons
ecosystem properties may change along wide distances in large lakes, a
fact well known from a global perspective.
 Lake Taihu, 2004:
Lake Taihu, 2004:
Fishing nets..
 Lake Taihu, 2004:
Lake Taihu, 2004:
Local Fishery.
 Lake Taihu, 2004:
Lake Taihu, 2004:
Net catch of fish.
 Lake Taihu, 2004:
Lake Taihu, 2004:
Ice-fish from lake Taihu served as a local
dish with scrambled eggs. Insert: Detail of ice-fish.
Lake Taihu extends over a distance of about 70 km from north to south and 50 km from east to west. The lake shoreline covers a length of about 465 km and is surrounded by urban districts as cities, villages and factories, farming areas and is partly even close to a mountain region. To meet these concerns of spatial heterogeneity in large Taihu, various sampling stations were used to continuously collect information from the lake (map of sampling stations shown Fig.1 in Chen et al. 2003 R, Fig.1 in Chen et al. 2003 R). Due to main nutrient enlargement in the north of the lake, the Meiliang Bay was sampled at various points in addition to lake centre stations. This bay is covering a surface area of about 100 km2, and is located near the city Wuxi. During the Taihu study period from 1991 to 1999, at both sites, the lake centre and Meiliang Bay, the biovolume of photosynthetic micro-organisms that are floating in the water column (phytoplankton), followed an increasing trend. The maximum peak of total phytoplankton biovolume was measured in August in the year 1998 in the Meiliang Bay (Fig.4 in Chen et al. 2003 R) and was about 118 mm3 L-1. Further, not only the phytoplankton biomass, but also the portion of prokaryotic photosynthetic organisms, i.e. of cyanobacteria (mainly of Chroococcales, a variety of Microcystis forms), increased with the years and were hence mainly responsible for the dramatic increase of phytoplankton biovolume in Meiliang Bay in 1998. The photos in the gallery 30-34/44 and photos in the text below are taken from a visit in 2000, two years later from the biomass peak recorded by sampling, but show in principle how such development of scum looks like. Water surface blooms of Microcystis are mainly built in summer and are lasting to early autumn in Taihu, as is common for summer-early autumn plankton in other eutrophied, shallow, well mixed lakes around the world. The reason for increasing development of Microcystis is the nutrient enrichment in lake Taihu over years (Figs.4&6 in Chen et al. 2003 R, Figs.3&4 in Chen et al. 2003 R). In the nineties, highest concentrations of total phosphorus and biovolume cyanobacteria were measured in the Meiliang Bay close to the inflow of tributaries and lower values of both at the lake centre (Table II & Fig.5 in Chen et al. 2003 R, Fig.2 in Chen et al. 2003 R). A similar situation of cyanobacterial blooms in shallow lakes is described for Old Danube S and Grosser Mueggelsee S on this website.
 Lake Taihu, 1994 & 1995:
Lake Taihu, 1994 & 1995:
Shrimp baiting traps in the
Meiliang Bay near the town Wutang. Bamboo shoots seen
above the water are connected with a bundle of old tea trees
(insert) at the lake bottom, which attracts freshwater shrimps as
habitat. Shrimps are harvested by washing and shaking the tea tree
bundles on the fisher boat. Also the 'macrophyte islands' seen
on the lake are shrimp baiting traps.
.
 Lake Taihu, 2004:
Lake Taihu, 2004:
Catch of small fish and
freshwater shrimps.
Some of these blooming
cyanobacteria
are known to produce various species of toxins, which harm the
ecosystem (e.g. harm aquatic animals from zooplankton to molluscs to
fish) but can also risk human health in case the lake water is used for
drinking, fishing and recreation (e.g. swimming). Therefore, in many
countries huge efforts are spent to monitor
and control the growth of cyanobacteria in such lakes,
mainly by forcing a sustained
reduction of external nutrient loading from the lake catchment
(e.g. construction of sewage plants).
Microcystis is easy
to identify when a scum of such cell material covers the shallow
littoral zone. After degradation of ‘green’ chlorophyll-a in dead cell
deposits of Microcystis
on a lakeshore, the main light-harvesting pigment, the phycobiline
phycocyanin, becomes unmasked. Therefore dead cell material of Microcystis
on a shore looks blue-green coloured. Phycocyanin is not a
common photosynthetic pigment among the phytoplankton species but only
specific for most cyanobacteria. Due to the turquoise-blue colour of
this pigment, these photoautotrophs are being called the
‘blue-green algae’. This
trivial name is still common, at least in popular science. In science
these organisms are called cyanobacteria
or cyano-prokaryotic taxa (the former cyanophyta, see more details on
cyanobacteria the next paragraph).
 Taihu
at Meiliang Bay, 2000:
Taihu
at Meiliang Bay, 2000:
Surface scum of cyanobacteria
('blue-greens') on lakeshore.
Such harmless looking ‘green spinach’ contains often harmful substances
built by Microcystis.
 Taihu at Meiliang Bay,
2000:
Taihu at Meiliang Bay,
2000:
Surface scum of cyanobacteria.
After degradation of green chlorophyll-a in dead cells of Microcystis, the turquoise
phycocyanin becomes un-masked and hence colors the scum blue-green.
 Catchment
of Taihu, at town
Wutang, 2000:
Catchment
of Taihu, at town
Wutang, 2000:
Heavy cyanobacterial blooms of
Microcystis color
the water greenish and are not only seen on the lake but also in nearby
channels. The photo is taken in early autumn.
.
 Lakeshore of Taihu, 2004:
Lakeshore of Taihu, 2004:
Macrophytes are planted on
floating platforms to utilize nutrients from the lake. This
bio-manipulation project aims primarily at reducing the nutrient
availability for phytoplankton growth. In addition, the
cultured plants on
these ‘artificial islands’ will be harvested as cut
flowers.
Due to the awareness of cyanobacterial blooms, monitoring studies and lake restoration management were aimed at controlling the growth of cyanobacteria in Taihu in recent years. A one side of bio-manipulation improving the water quality is illustrated by photos 45/47-50 in the gallery and text showing plantations of wild macrophytes and flowers in Taihu. An example of internal lake restoration (reduction of phosphate release from sediment) is described in greater detail on this website for the small shallow lake Old Danube S.
sampling cyanobacteria in a lake: the difficulty to quantify properly the biovolume of these colony-forming micro-organisms.
Phytoplankton samples are usually taken at the deepest site in the case of a small water body (see sampling along a vertical profile in deep alpine lake Mondsee) or at several stations for a large lake. In case of large Taihu, two centre stations and five stations in Meiliang Bay were sampled during the period 1991 to 1999, taking account the spatial heterogeneity in the lake mentioned above. Such a large number of sampling stations is most appropriate being aware of the patchiness of planktic microorganisms in a lake. It can be difficult to take representative phytoplankton samples on a calm day as phytoplankton cells are usually not evenly distributed over the whole lake but form rather under-water ‘cloud-formations’ floating horizontally in the water. In the case of cyanobacteria, it becomes even more delicate, as these microorganisms are able to stratify vertically by cellular buoyancy regulation (see further depth distribution of Planktothrix in Mondsee). In a calm period, cyanobacteria typically such as Microcystis in Taihu, stratify for some hours at the top surface or near surface water layer. Samples taken with a Ruttner-sampler in the top 0.5m layer, relate to the high cell density of cyanobacterial surface scum and may therefore overestimate the cyanobacterial biomass for the lake. In turn Ruttner-samples taken at 0.5m-1m below the surface might tend to underestimate the cell density of cyanobacteria in the water column. Accordingly, a representative sample for cyanobacteria in a shallow lake is suggested to be taken by ‘tube sampling’. The sampler is a tube of 1. 20m-length and 8cm-diameter, made of plexiglass (acrylic glass) and is operated like a sediment corer but for vertical sampling of the top 1.20m water column. The water sample is stored in the tube by a plug-in the form of a rubber stopper on the top opening, transferred to a sampling container and drained at the bottom opening by removing the rubber stopper on top.
 Taihu,
shore at Meiliang Bay,
autumn
2000:
Taihu,
shore at Meiliang Bay,
autumn
2000:
The 'blue-greens' on the lakeshore are easy to identify
due to the
turquoise colured dead cells of Microcystis
that are are still containing the pigment
phycocyanin.
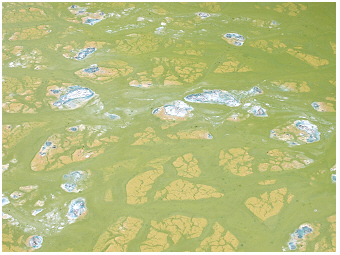 Taihu, lakeshore surface
scum of Microcystis
at Meilaing Bay,
autumn 2000:
Taihu, lakeshore surface
scum of Microcystis
at Meilaing Bay,
autumn 2000:
The cells of the green material might be alive with
vital pigment composition. Those of yellow colour are already dead
with partly degraded chlorophyll-a. The blue materials are dead
cells
that still contain phycocyanin as mentioned before - the white material
consists of dead cells with totally degraded pigments.
Depending on the wind direction, cyanobacterial blooms can be moved to a lagoon or bay. Such wind-driven accumulation of cyanobacterial cells often results in an eye-catching scum formed on shallow near-shore areas, as e.g. observed at the shoreline of Meiliang Bay. The cell-density of cyanobacterial cells in the near-shore scum sample is quantitatively not representative for an open-lake water sample but overestimates cyanobacterial biovolume in the lake. Even through it is difficult to quantity of cyanobacterial biomass for the lake by a shore-sample, such near-shore scum gives a clear signature for a huge mass-development of cyanobacteria in the lake (see also surface scum for red coloured cyanobacteria in the deep alpine lake Mondsee). The bio-gradation of dead cell material of cyanobacterial scum can be seen by the colour changing from yellow-green to turquoise-blue. This blue colour is due to the specific photosynthetic pigment phycocyanin in Microcystis cells (delayed fluorescence excitation spectra of phycocyanin-rich cyanobacteria are shown in Fig.6 in Greisberger & Teubner 2007 R). As long as Microcystis cells are alive, the blue-green colour of phycocyanin is masked by the green-colour of photosynthetic chlorophyll-a. The trivial name ‘the blue-greens’ refers to this blue colour of dry cyanobacterial cells commonly seen on shorelines of nutrient-rich lakes. In case the Microsystis strains at the shore were toxic forms, with dead cells, not only the colour of the cells changes but also high concentrations of toxins are released from the cells to the water or shoreline. In many lakes the cyanobacterial toxin concentration is highest when cyanobacterial blooms break down, usually in late summer to early autumn (often the summer species growth extends to autumn and autumn species even occur alraedy in lower abundance in summer - see the two principal seasons of phytoplankton composition a year described for mainly shallow lakes in Berlin-Brandenburg, the northern part of Germany, on this website S). For this reason, cyanobacterial blooms are most harmful during the time from August to September. The bio-degradation of cyanobacterial toxins of summer blooms is lasting much longer than for common cell material as e.g. chlorophyll of these microorganisms.
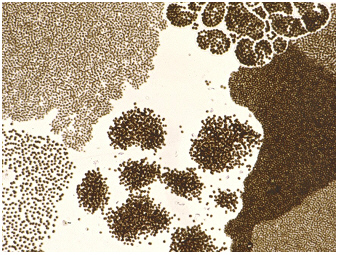 Microcystis
colonies sampled from Taihu, 2000:
Microcystis
colonies sampled from Taihu, 2000:
In natural phytoplankton not only a single taxon of Microcystis but many taxa of
this genus occur in a plankton sample (Table
III
in Chen et al.
2003 R).
The various types of Microcystis
can be identified
under the light microscope, e.g. by the pattern of cell arrangement,
the form of cell aggregates, the type of enveloping mucilage and the
cell size.
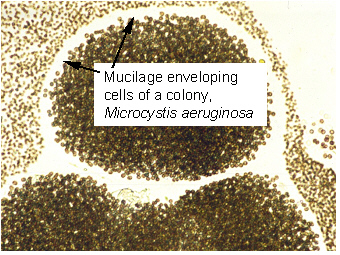 Microcystis
colonies sampled from Taihu, 2000:
Microcystis
colonies sampled from Taihu, 2000:
A mucilage envelope that can be easily seen under the light microscope
separates the cell aggregates of neighboring colonies. In case cells
are separated by lab treatment for a simple counting procedure (see
determining the biovolume of cyanobacteria the text below), the
different taxa of Microcystis
cannot be further identified, as the information about the
characteristics of the colonies is lost.
Estimating the quantity of cyanobacteria by a counting procedure under the light microscope is even tricky in the lab. Many cyanobacteria, as e.g. Microcystis, are colony-forming microorganisms. The spherical cells are embedded in mucilage forming cell clusters. The arrangement of cells and the type of mucilage margin discriminate mainly between various eco-morphotypes/morphospecies of Microcystis. The cells can be irregularly dispersed (e.g. Microcystis aeroginosa, M. flos-aquae) or non-randomly arranged in one to two layers (e.g. M. wesenbergei) in a colony. Some colonies even form sub-colonies of three-dimensional cube-arrangements of cells (e.g. M. viridis). The margin of mucilage can be far distant from the cluster of cells and almost invisible (e.g. M. aeroginosa, M. flos-aquae), or easy to identify due to refracting properties (e.g. M. wesenbergei). In some cases, the margin of mucilage is narrow to the cells, envelopes directly the cell surface and hence looks like an undulated line (M. viridis) under the microscope. Some of these Microcystis eco-types are known to occur in nutrient-poor rather than in nutrient-rich lakes and vice versa; some are commonly known to produce higher toxin concentrations than others.
For estimating phytoplankton quantity not only the biovolume but also the concentration of chlorophyll-a in a phytoplankton sample is measured. Chlorophyll-a is a ubiquitous light harvesting pigment that occurs in all photosynthetic micro-organisms from eukaryotic algae to cyanobacterial forms. Therefore, both the biovolume and the chlorophyll-a concentration of a phytoplankton sample might correspond to each other. In a phytoplankton with a balanced contribution among many eukaryotic and some pro-karyotic species – as it is a common phytoplankton situation in natural freshwater lakes – the both measures fit well to each other. In some particular cases of phytoplankton development, however, the phytoplankton biovolume or biomass can be underestimated or overestimated by measures of chlorophyll-a. The reason is the specific pigment composition among phytoplankton taxa. Different from many eukaryotic planktonic algae, the cyanobacterial cells contain in addition to chlorophyll-a further main light harvesting pigments, namely phycobilines as e.g. phycocyanin. Therefore, light harvesting in cyanobacteria is not only accomplished by chlorophyll-a but also by phycobilines. Their photosynthesis is hence adapted to a relative low cellular concentration of chlorophyll-a. The opposite is with chlorophytes as these eukaryotic species are, in particular, ‘chlorophyll-a rich’ organisms. Taihu provides an example that years of peaking annual phytoplankton correspond not necessarily to years with highest annual chlorophyll-a concentration and vice versa. Year 1997 is the peak year for biovolume (see total phytoplankton for Meiliang Bay in Fig.4A in Chen et al. 2003 R), but years 1995 and 1996 are the years of highest chlorophyll-a measurements (see chl-a for Meiliang Bay in Fig.6A in Chen et al. 2003 R). The species composition from 1997 was different from those two years before. In 1997, the relative contribution of ‘chlorophyll-a poor’ cyanobacteria (Microcystis) was much higher than the two years in the past. In addition, ‘chlorophyll-a-rich’ chlorophytes occurred only in 1995 and 1996, but disappeared in 1997. Further details about specific pigment relations in cells and the physiological adjustment to cellular chlorophyll content are described e.g. in Teubner et al. 2001 R and Teubner & Greisberger 2007 R.
Identifying the eco-morphotypes of various Microcystis strains can provide valuable information useful for an ecosystem assessment. To determine the Microcystis biovolume (or biomass) of in a water sample via microscopy, the number of all cells of a certain small sub-sample volume is counted and is multiplied with the mean volume of the spherical cells. Counting crowded cells in colonies under the light microscope, however, might be a confusing lab procedure. Also, statistical validation for the counting results due to the large size of colonial formations can be somewhat challenging. Therefore, common counting procedures for Microcystis suggest separating cells from the mucilage before counting. For this purpose, lake samples are slightly warmed up (e.g. by short exposure to sun-light) or treated with ultrasonic. The disadvantage of such single-cell-counting procedure is, that information about the quantity of different eco-morphotypes, recognised by the cell arrangement and type of mucilage, is lost. Both, the identification of Microcystis eco-morphotypes/morphospecies in various habitats on the one side (counting of cells embedded in colonies), and advances of molecular characterization on the other, might be considered necessary to understand the ecology of these photosynthetic prokaryotic microorganisms.
cited References: about taihu
Liu X, Teubner K, Chen Y (2016) Water quality characteristics of Poyang Lake, China, in response to changes in the water level. Hydrology Research, in press DOI:10.2166/nh.2016.209 OpenAccess
Greisberger S, Teubner K (2007) Does pigment composition reflect phytoplankton community structure in differing temperature and light conditions in a deep alpine lake? An approach using HPLC and delayed fluorescence (DF) techniques. Journal of Phycology, 43:1108-1119 doi:10.1111/j.1529-8817.2007.00404.x Look-Inside FurtherLink
Chen Y, Qin B, Teubner K Dokulil MT (2003) Long-term dynamics of phytoplankton assemblages: Microcystis-domination in Lake Taihu, a large shallow lake in China. Journal of Plankton Research, 25 (1):445-453 Abstract Abstract in Chinese OpenAccess
Chen Y, Fan C, Teubner K Dokulil MT (2003) Changes of nutrients and phytoplankton chlorophyll-a in a large shallow lake, Taihu, China: an 8-year investigation. Hydrobiologia, 506:273-79 Abstract OpenAccess
Teubner K, Sarobe A, Vadrucci MR, Dokulil M (2001) 14C photosynthesis and pigment pattern of phytoplankton as size related adaptation strategies in alpine lakes. Aquat Sci 63: 310-25. doi:10.1007/PL00001357 Look-Inside FurtherLink
Dokulil M, Teubner K (2000) Cyanobacterial dominance in lakes. Hydrobiologia 438:1-12 Abstract FurtherLink
